As of 2023, there is a new Study Design for VCE Chemistry Units 1&2. Confused? Not sure what this means for you? Need a quick summary of the major changes? Have no fear! ATAR Notes is here to explain everything you need to know!
Why does this matter?
The Study Design is the most important document for your VCE subjects. It contans a dot point list of everything you need to know for both the Units 1&2 and Units 3&4 courses. If you haven't looked at the VCE Chemistry Study Design, do that ASAP! But don't worry if it's a bit overwhelming at first – we'll go through the important bits for you!
Your teacher will use this Study Design to create all of your lessons and assessment tasks this year. And next year, all of your SACs and the final Units 3&4 exam will also be based on the Study Design. So this document is basically a checklist for you to tick off as you cover each concept in class.
However... this document is primarily written for teachers, not students. So it contains a bunch of irrelevant stuff you don't need to know.
The fact that this is a new Study Design for 2023 also means there's going to be a bit more confusion amongst both teachers and students about the scope of certain dot points and what, precisely, is assessable.
That's why we've put together this article – to explain and demystify the Chemistry Study Design for you!
How does this affect me?
The new Chemistry Study Design has staged implementation, which means it takes affect for Units 1&2 first, and then Units 3&4 the year after. In simple terms:
• If you are completing Chem 3&4 in 2023, this DOES NOT AFFECT YOU! You will use the current 2016–2023 Study Design (so stop reading this article!).
• If you are completing Chem 1&2 in 2023, you will be the first new year level to do the NEW STUDY DESIGN! (Congratulations, guinea pigs!!) You'll then complete Chem 3&4 in 2024 under this new Study Design.
• If you are completing Chem 1&2 or 3&4 in 2024 and beyond, you will use the new Study Design.
So what's been added?
The structure of the Study Design has changed somewhat, so let's break it down into the important sections:

We're going to skip all the introductory material as that's only relevant to teachers. Let's jump straight to page 11 to the Cross-study specifications. If you're doing another VCE Science subject (Bio, Physics, or Psych) then these may already be familiar to you, as they're fairly similar for each subject. This section breaks down the key info for your experimental design/practical investigation tasks, including all of the skills you could be asked to demonstrate. Most of these are the same as (or slightly improved versions of) the ones listed in the old Study Design, though there are a couple of new points. For example:
· apply sustainability concepts (green chemistry principles, development goals and the transition from a linear towards a circular economy) to analyse and evaluate responses to chemistry-based scenarios, case studies, issues and challenges
· identify and explain when judgements or decisions associated with chemistry-related issues may be based on sociocultural, economic, political, legal and/or ethical factors and not solely on scientific evidence
This first dot reflects the new focus on sustainability, which we'll explain in more detail below. The second dot point tells you that even though this is a science subject, you'll need some humanities skills when applying your knowledge. VCAA will likely use those factors to add a layer of difficulty to questions, so don't be surprised if you see something like this on your SACs and exams!
What is green chemistry?
'Green chemistry' is the name given to concepts of environmental sustainability for chemical processes. This is a very popular field of study that you may go on to pursue at university, but for VCE purposes, there are two key concepts we need to know: green chemistry principles and the linear vs. circular economy.
The Study Design lists 7 principles you need to understand and remember. These are things we need to consider when designing reactions or chemical processes to ensure we are minimising negative environmental impacts.
1. Atom economy: Processes/pathways should be designed to maximise incorporation of all reactant materials used in the process into the final product.
2. Catalysis: Catalysts should be selected to generate the same desired product(s) with less waste and using less energy and reagents in reaction processes/pathways.
3. Design for degradation: Chemical products should be designed so that at the end of their use they break down into harmless degradation products and do not persist in the environment.
4. Design for energy efficiency: Processes/pathways should be designed for maximum energy efficiency and with minimal negative environmental and economic impacts.
5. Designing safer chemicals: Chemical products should be designed to achieve their intended function while minimising toxicity.
6. Prevention of wastes: It is better to prevent waste than to treat or clean up waste after it has been produced.
7. Use of renewable feedstocks: Raw materials or feedstocks should be made from renewable (mainly plant-based) materials, rather than from fossil fuels, whenever practicable.
In reality, there are actually 12 green chem principles, but you don't have to know about those extra ones. Below is a graphic of all 12 with the irrelevant ones greyed-out.
Source: adapted from Green methodologies for the synthesis of 2-aminothiophene
In line with the principles above, you also need to understand the difference between a linear model of chemical production (i.e. take resources, make a thing, use a thing, dispose of the thing) and a circular model (i.e. repurpose/reuse/recycle things) with reference to natural resources and materials.
These are fairly intuitive concepts: a linear economy goes in a straight line, starting with resources and ending in waste. By contrast, a circular economy involves recycling and repurposing materials as much as possible to minimise the environmental impacts of waste.
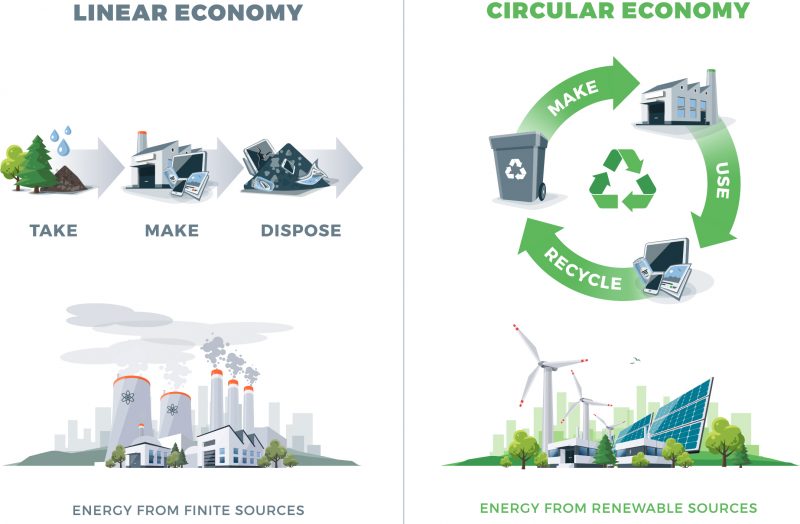
Source: https://twosidesna.org/US/paper-and-the-circular-economy/
Now that we've covered the cross-study terms and key skills, we can scroll down the Study Design to the Unit 1 section. This is where we reach the all-important key knowledge dot points!
For a quick overview of the changes, here's our expert tutor Josh who will run you through the Study Design and summarise what's new.
(Also, go subscribe to our YouTube channel to catch more videos like this for all of your VCE subjects!)
New stuff in Unit 1
Below is a breakdown of the new topics that have been added to the course. You won't find any information or practice questions about these concepts in old textboooks or past exams, but luckily these are all highly relevant and important things for real-world chemistry, so you'll find a decent amount of info for these online. We'll include some recommended links for the first few to get you started.
Also note that we're not covering every dot point on the Study Design, only the brand new ones. To ensure you cover everything, you should still download a copy of the Study Design for yourself!
Area of Study 1
• Circular economy and metal recycling
• Solubility and precipitation reactions (partially from old Unit 2 AOS 2)
• Ionic equations (partially from old Unit 1 AOS 2)
Area of Study 2
• Relative atomic mass
• Plant-based biomass for everyday products
• Polymer plastics (addition vs. condensation reactions, and linear vs. circular economy)
• Fossil fuel plastics vs. bioplastics
Note that Area of Study 3 is a list of four option topics, of which you or your teacher will choose one.
New stuff in Unit 2
Area of Study 1
• Neutralisation reactions and antacids
• Calculating pH of acids and bases
• Acid rain and ocean acidity
• Applications of redox chem (e.g. corrosion, simple primary cells)
Area of Study 2
• Precipitation reactions to remove water impurities (partially from old Unit 2 AOS 1)
• Greenhouse gases and the greenhouse gas effect
• The ideal gas equation
• Stoichiometry
What's been removed?
Below is a list of all the topics that have been completely taken out of the Chemistry 1&2 course. If you see any of these words in an old textbook or past exam, you can safely skip and ignore them! You don't need to know anything about these, but it may be worth writing this list down somewhere so that you can consult it if you're ever going through old practice questions and aren't sure what's relevant.
❌ Nanoparticles and nanomaterials
❌ spdf notation of electron configurations
❌ Bohr and Schrodinger models
❌ Alloy metals
❌ Origins of crude oil
❌ Solvent properties of water
❌ Water sampling protocols and chemical/organic contaminants
❌ Gravimetric analysis, atomic absorption spectroscopy (AAS), and high performance liquid chromatography (HPLC)
❌ Sources of acids/bases in water (e.g. mining activity, industrial waste)
And that's everything you need to know! Remember to grab a copy of the Chem Study Design and use those key knowledge dot points as a checklist throughout the year. And stay tuned for more VCE Chemistry resources on ATAR Notes! ✌️ 🧪 👨🔬






